Director: Mario A. Bianchet, PhD. Office: WBSBS 608B
Located in 719E , 623 WBSB.
Our iLab account is live! Users must have an iLab account to use the facility. Please see below for information on equipment and the list of user fees.
For information, please contact Mario A. Bianchet, Ph.D., (410) 614-8221 or e-mail to bianchet@jhmi.edu.
We have an array of instruments that are available for use by members of the Johns Hopkins community as well as by outside users. Equipment includes:
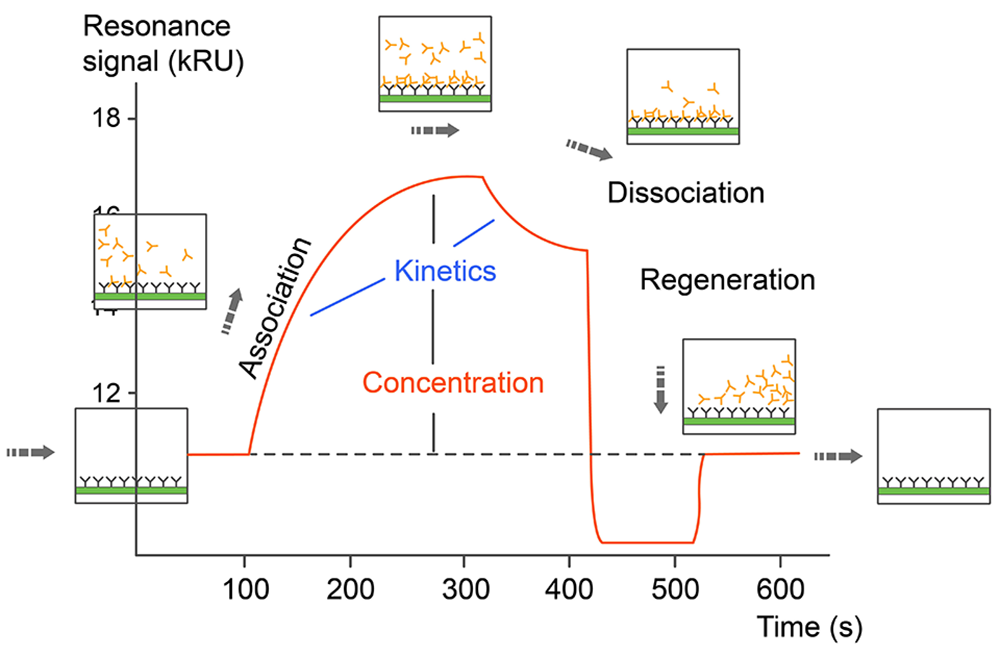
Surface Plasmon Resonance (SPR) interaction analysis equipment (Biacore 8K) will provide a label-free detection method for studying the binding behavior of biomolecules. The Cytiva™ Biacore 8K is a high-throughput and high-sensitivity SPR system that delivers binding data of outstanding quality. Samples are immobilized on a chip surface using one of several available chemistries while both binding and dissociation of another molecule are monitored. This method can be used to study a wide array of binding interactions.
Unassisted use of the instrument will be allowed after a short local training about local standard procedures are taken by the user a on-line curse provided by Cytiva is strongly recomended. The Biacore training is required, and the cost is $269 per registration for academics (as 12/2022). To register for Cytiva training click here . To take the core training please send an e-mail to core. Standard operating procedures document could be access at SOP.
Mass photometry (MP) is a revolutionary new label-free method for accurately measuring the mass of single macromolecules in solution. MP builds upon the principles of interference reflection microscopy and interferometric scattering microscopy. As a result, the light scattered by single molecules can be reliably detected and correlated to their molecular mass. The Refeyn® TwoMP™ mass photometer provided by the facility will allow the characterization of the biomolecular samples, their oligomerization, biomolecular interactions, and macromolecular assemblies.


Unassisted use of the instrument will be allowed after a short local training about local standard procedures is taken by the user. To take the local training please make a request training in Ilab.
Handouts:
MicroScale thermophoresis (MST) – NANOTEMPER® Monolith™ NT.115. This instrument allows a straightforward, fast, and precise approach to quantifying biomolecular interactions. MST is the exception to the label-free techniques instruments of the core. One of the molecules must be fluorescently tagged. Its advantage is that it allows the detection of a broader range of interaction types, ranging from ion and fragment binding up to interactions of macromolecular complexes such as liposomes or ribosomes. MST has become a standard technique to detect specific macromolecule-probe interactions. This method measures the differences in the movement rate through a microscopic temperature gradient caused when complexes are formed. MST can be applied to characterize any biomolecular interaction, including protein-DNA, protein-RNA, protein-protein, and antigen-antibody interactions, as well as ligand binding to ternary complexes.
Unassisted use of the instrument will be allowed after a short local training about local standard procedures is taken by the user. To take the core training please request training in iLab .
Size Exclusion Multi-Angle Light Scattering (SEC-MALS) with a dedicated angle for Dynamic Light Scattering (DLS): SEC-MALS combines size-exclusion chromatography with multi-angle light scattering to determine the weight averaged molar mass (Mw) of macromolecules/polymers/bioconjugates in solution as they are separated by SEC without the need of column calibration standards.
Instrumentation:
Wyatt DAWN® HELEOS-II™ + QELS DLS module
Wyatt Optilab® T-rEX Refractive Index Detector- for Concentration Determinations
Agilent 1260 ISO PUMP
Agilent 1260 DAD WR Diode Array Detector
Agilent Manual Injector (maximum 100uL)
Column:WTC-050S5; 5uM particle; 500A pore; 7.8X300mm, Hydrophilic Macromolecules; 15,000 to 5,000,000 Da
This UV-MALS-RI triple-detection system combined with Wyatt’s Astra software allows analysis of copolymers and protein conjugates (glycoproteins, PEGylated proteins, surfactant-bound membrane proteins, etc.) The resulting calculations give you the absolute molar mass of the conjugate as well as the individual components. The addition of the Wyatt QELS DLS module enables radius of hydration (Rh) calculations across the peak.
Unassisted use of the instrument will be allowed after a short local training about local standard procedures is taken by the user. To take the local training please rquest training in iLab .
Documents:
Isothermal titration calorimetry (ITC)
MacBio core will provide two different choices of calorimeters: an ITC VPITC and a Malvern Analytics ITC200, which handles smaller sample sizes (200 µl). ITC is the gold standard for quantitating molecules’ interactions and is used to study a wide range of biomolecular interactions. Applications range from drug design to fundamental research, such as understanding and regulating signal transduction pathways.
Standard Operating Procedure (SOP)
Differential Scanning Calorimetry (DSC) is an analytical technique for characterizing the thermal stability of biomolecules. It does this by measuring the heat change associated with the molecule’s thermal denaturation when heated at a constant rate. DSC is also used to determine the change in heat capacity. DSC can elucidate the factors contributing to the folding and stability of native biomolecules. The facility has a Malvern Microcal VP-CAPILLARY DSC Micro Calorimeter. These systems deliver direct, label-free, and in-solution measurements of binding affinity and thermodynamic parameters in a single experiment.
NanoDSC AUTOSAMPLER with Interface P/N 602215.
Nano DSC is a high-resolution method for quantifying the thermal stability of large biomolecules and biotherapeutics. This instrument measures the heat of reaction from tertiary and secondary structure changes that occur when a biomolecule unfolds, or melts in the case of nucleic acids and lipids. The Nano DSC provides peak temperature when unfolding occurs and a total heat from the unfolding event. (tainstruments, 2024)
Crystallization and X-ray resources.
X-ray resources. The core facilitates a wide range the crystal freezing instrumental, crystal shipping pucks (NSLS, and Argon styles), and shipping dewars. Also a synchrotron mail-in remote X-ray data collection through a JHU-wide consortia managed by the core director is available to university PIs.
Tray-setting robot. The SPT Labtech Mosquito® LCP is a liquid-handling robot that can set up 48- and 96-well crystallization trays using nanoliters of protein solution per drop. The instrument uses positive displacement pipetting using disposable micropipettes with individual pistons to deliver exceptional accuracy and precision, irrespective of liquid class. In addition, the instrument allows accurate, reproducible automation for liquid cubic phase (LCP) setups of 20 nl.The Mosquito LCP offers a fully automated and highly accurate solution for setting up LCP screens, complete with an optional LCP mixer. With this device, you can dispense lipidic cubic phase in nanolitre volumes as low as 25 nL, using a microsyringe dispenser. The LCP drops are then immediately covered with screen solution using Mosquito’s standard disposable pipette, ensuring precise and efficient results. Be aware that UV transparent boxes and substrates are required to be using the UV imaging resources of the core ( see below)
Automatic imaging of crystal trays. Two Formulatrix Rockimagers ® are available to automatically records images of crystallization drops while maintaining crystal trays at a constant temperature. High optical resolution is achieved by increasing the numerical aperture of the objective and combining multiple slices into one Extended Focus Image. The instrument also facilitates record-keeping for the barcoded crystal trays. Multiple images of each drop can be captured with user-adjustable settings, including exposure, polarization, and condenser aperture.
RATES
| Instrument | units | Price JHU | JHU Partners | Non-profit | For-profit |
| SPR (Biacore 8K) | hours | $ 25.00 | $ 30.00 | $ 25.00 | $ 43.00 |
| Mass Photometer (Two-MP Refeyn™) | hours | $ 15.00 | $ 18.00 | $ 15.00 | $ 26.00 |
| MST | titration | $ 30.00 | $ 36.00 | $ 30.00 | $ 52.00 |
| SEC-MALS | 1/2 day | $ 70.00 | $ 84.00 | $ 70.00 | $ 121.00 |
| Crystallization robot (Mosquito-LCP®) | tray | $ 5.00 | $ 6.00 | $ 5.00 | $ 9.00 |
| Automatic Imaging (RockImager®) | tray | $ 5.00 | $ 6.00 | $ 5.00 | $ 9.00 |
| ITC/DSC | hours | $ 25.00 | $ 30.00 | $ 25.00 | $ 43.00 |
| Biacore 8K, assisted use | hours | $ 90.00 | $ 108.00 | $ 90.00 | $ 155.00 |
| Mass photometer, assisted use | hours | $ 90.00 | $ 108.00 | $ 90.00 | $ 155.00 |
| MST, assisted use | titration | $ 150.00 | $ 180.00 | $ 150.00 | $ 258.00 |
| SEC-MALS, assisted use | sample | $ 250.00 | $ 300.00 | $ 250.00 | $ 430.00 |
| Crystallization robot assisted use | tray | $ 30.00 | $ 36.00 | $ 30.00 | $ 52.00 |
| RockImager® assisted used | week-tray | $ 30.00 | $ 36.00 | $ 30.00 | $ 52.00 |
| ITC/DSC, assisted use | hours | $ 45.00 | $ 54.00 | $ 45.00 | $ 78.00 |
| Training on Biacore 8K | Training (3h) | $ 150.00 | $ 180.00 | $ 150.00 | $ 258.00 |
| Training on Mass Photometer | Training (1h) | $ 50.00 | $ 60.00 | $ 50.00 | $ 86.00 |
| Training on MST | Training (1h) | $ 100.00 | $ 120.00 | $ 100.00 | $ 172.00 |
| Training on SEC-MALS | Training (3h) | $ 200.00 | $ 240.00 | $ 200.00 | $ 344.00 |
| Training on Crystallization or Imager robot | Training (1h) | $ 50.00 | $ 60.00 | $ 50.00 | $ 86.00 |
| Training ITC/DSC | Training (1h) | $ 50.00 | $ 60.00 | $ 50.00 | $ 86.00 |